Thank you for your interest in Fomae’s CyborGel®. There is currently a “Treatment Gap” with no reliable treatment options for early osteoarthritis to predictably forestall or prevent progression towards the clinical need for a total joint replacement.
Formae's CyborGel Synthetic Cartilage Implant System,
FDA Designated Breakthrough Device


CyborGel® has been engineered to mimic and reproduce the structure, microanatomy, and functional properties of natural joint cartilage. It is being investigated for a minimally invasive procedure (Arthroscopy) with the goal of supporting a rapid recovery that would enable patients to walk unassisted the same day of installation.

⦾ Help resurface damaged articular cartilage with our Biomimetic CyborGel® implant system, with the goal of functional joint restoration.
⦾ Help provide pain relief from arthritic bone-on-bone wear.
⦾ Help reduce progressive deterioration of synovial joints, thereby supporting to reduce the clinical need for Total Joint Replacement.

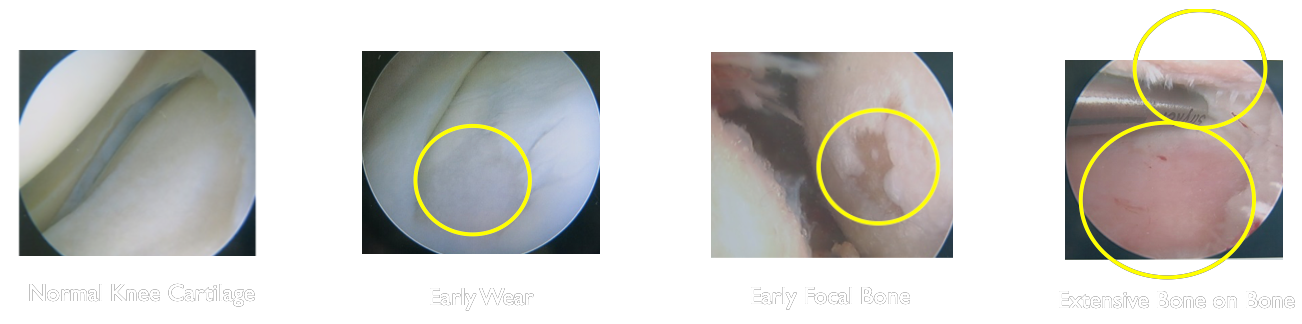
Osteoarthritis impacts the everyday quality of life for 20-40 Million Americans and around 500 Million people worldwide (7% of the global population). However the standard of care has not significantly changed in over 40 years.
Patients are encouraged to manage their pain with painkillers or steroids until the progressive degeneration of the disease necessitates a Total Knee Replacement, an invasive procedure that sets patients back upwards of $50K and a 6 to 12 week (and potentially longer) recovery period.
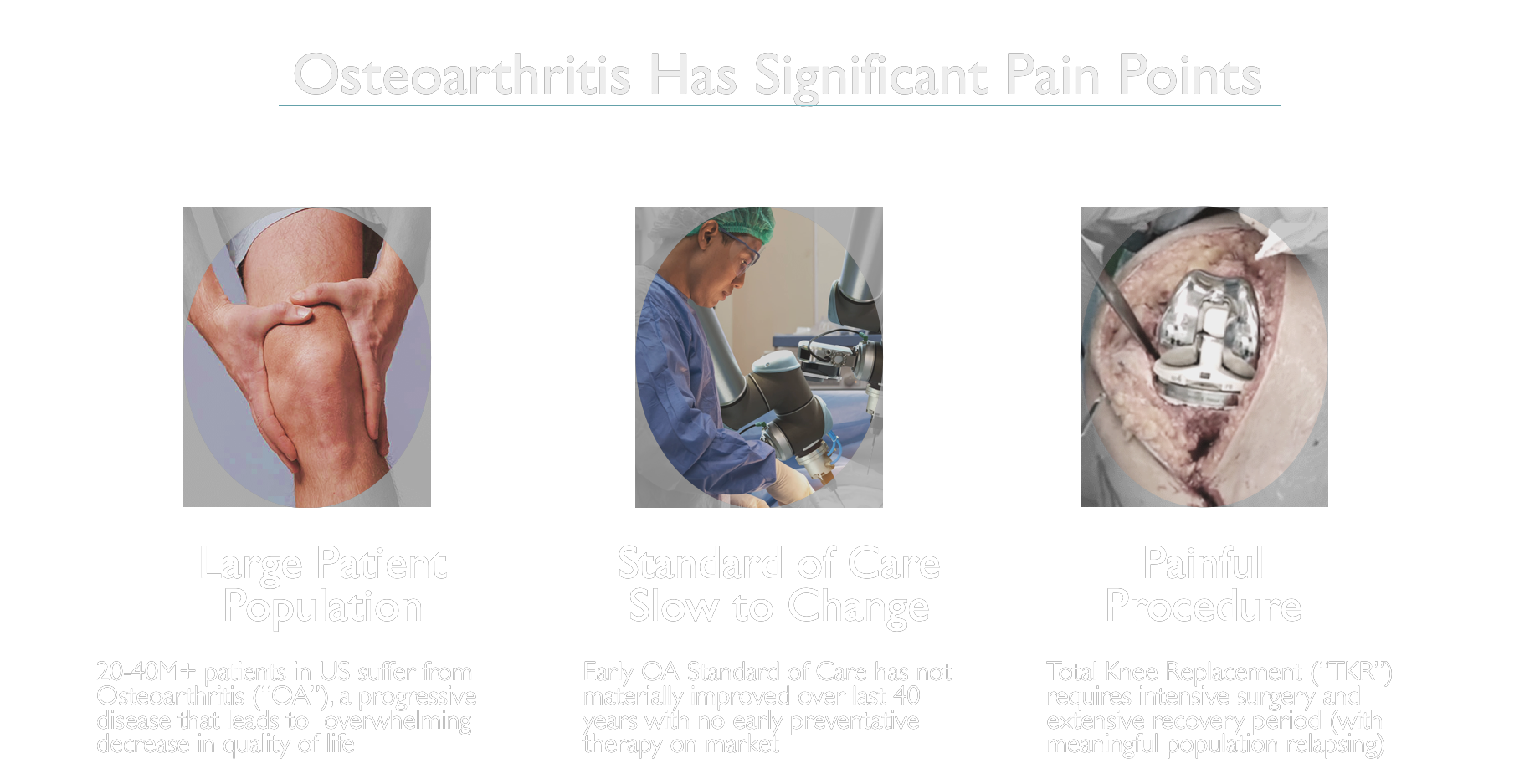

It’s no surprise that upwards of 2 Million American patients are delaying treatment every year, and with good reason. There is a huge gap in Early Intervention and Preventative Treatment options for patients suffering with OA. We’ve identified this need and believe our patented Arthroscopic Joint Resurfacing implant, CyborGel®, is being studied as the potential solution.
Thank you for your interest in Fomae’s CyborGel®. There is currently a “Treatment Gap” with no reliable treatment options for early osteoarthritis to predictably forestall or prevent progression towards the clinical need for a total joint replacement.
Formae's CyborGel Synthetic Cartilage Implant System,
FDA Designated Breakthrough Device
CyborGel® has been engineered to mimic and reproduce the structure, microanatomy, and functional properties of natural joint cartilage. It is being investigated for a minimally invasive procedure (Arthroscopy) with the goal of supporting a rapid recovery that would enable patients to walk unassisted the same day of installation.

The ultimate goals of this joint sparing procedure are to:
⦾ Help resurface damaged articular cartilage with our Biomimetic CyborGel® implant system, with the goal of functional joint restoration.
⦾ Help provide pain relief from arthritic bone-on-bone wear.
⦾ Help reduce progressive deterioration of synovial joints, thereby supporting to reduce the clinical need for Total Joint Replacement.

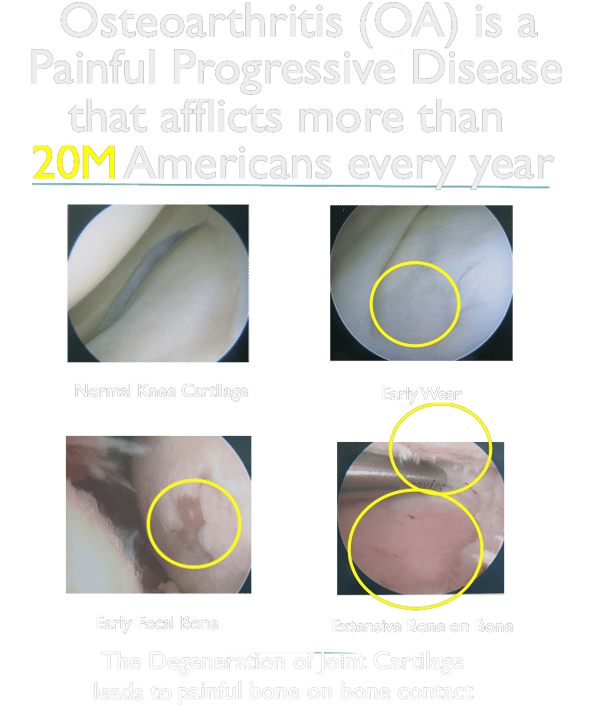
Osteoarthritis impacts the everyday quality of life for 20-40 Million Americans and around 500 Million people worldwide (7% of the global population). However the standard of care has not significantly changed in over 40 years.
Patients are encouraged to manage their pain with painkillers or steroids until the progressive degeneration of the disease necessitates a Total Knee Replacement, an invasive procedure that sets patients back upwards of $50K and a 6 to 12 week (and potentially longer) recovery period.


Breakthrough Biomimetic Cartilage Implant System for OA
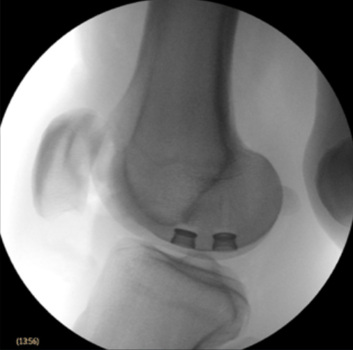
Our CyborGel® biomimetic implant system utilizes a 3D printed Trabecular Porous Metal Anchor, designed to support osseointegration (bone ingrowth) for stable bone-implant fixation. Our hydrogel synthetic cartilage has endured over 10M cycles (and counting) without progressive wear. The average person walks 1M cycles in a year.

*GLP Mode I Knee Simulated Tribology:
CyborGel Synthetic Cartilage hits 10M cycles without progressive wear.

Initial testing indicates the potential for a long term solution to OA. While testing is ongoing, the durability of our CyborGel implants suggests the potential to be a long-term solution to Osteoarthritis of the knee and to help delay the need for a Total Knee Replacement.


The 1.7M patients actively delaying TKR indicate a significant market need for a Early Intervention treatment option for OA, even before we consider the patients undergoing a TKR (~800,000 / yr) that would be thrilled to have an alternative. At a $5K ASP, those 1.7M convert to a potential $8.5B Market. If we expand to include potential forays into Veterinary Medicine and ultimately the expansion of CyborGel techonology to address OsteoArthritis of all joints (the shoulder, hip, wrist, and ankle), the opportunities are substantial.


To learn more in depth about Formae's research and business projections, please join our mailing list for investors.

Our CyborGel® biomimetic implant system utilizes a 3D printed Trabecular Porous Metal Anchor, facilitating osseointegration (bone ingrowth) for stable bone healing fixation. Our hydrogel synthetic cartilage has endured over 10M cycles (and counting) without progressive wear. The average person walks 1M cycles in a year.


*GLP Mode I Knee Simulated Tribology:
CyborGel Synthetic Cartilage hits 10M cycles without progressive wear.

A ten year solution to OA is already viable for the marketplace. But testing is ongoing and our CyborGel implants are holding strong. This means CyborGel could very well be a permanent solution to Osteoarthritis of the knee and delay the need for a Total Knee Replacement indefinitely.


The 1.7M patients actively delaying TKR indicate an immediate marketplace viability for CyborGel as a revolutionary Early Treatment Option for OA, even before we consider the patients undergoing a TKR (~800,000 / yr) that would be thrilled to have an alternative. At a $5K ASP, those 1.7M convert to a $8.5B Market. But if we zoom out further to include potential forays into Veterinary Medicine and ultimately the expansion of CyborGel technology to address OsteoArthritis of all joints (the shoulder, hip, wrist, and ankle), well this is just the start.







